

This process activates the unfolded protein response (UPR), which resolves the stress by reducing the overall protein synthesis, increasing the capacity for protein folding, and promoting the removal of misfolded proteins by the ER-associated degradation (ERAD) ( Travers KJ et al. Unfolded or misfolded proteins can cause ER stress by accumulating in the lumen.

Proteins that are targeted for other parts of the secretory pathway, the plasma membrane or other organelles begin the process of transport from the ER, using exit sites present in the transitional ER.Ĭontrol mechanisms ensure that only correctly folded proteins are transported out of the ER. The ER lumen contains proteins that mediate proper protein folding, post-translational modifications and quality control of the newly synthesized proteins. If a transmembrane domain is present, the protein gets incorporated in the lipid bilayer of the ER. After docking to the ER, translation continues and the nascent protein gets translocated across the ER membrane through channels referred to as translocons. Translation of these proteins is initiated in the cytosol, but the emergence of an N-terminal signal peptide leads to binding of a complex known as the Signal Recognition Particle (SRP), which then guides the nascent protein and the ribosome to SRP receptors in the rough ER. One of the major functions of ER is in translation of mRNA to certain groups of proteins, including secreted proteins and integral membrane proteins, but also some cytosolic proteins. The ER is known to serve multiple roles in human cells ( Schwarz DS et al. The function of the endoplasmic reticulum The morphology of the ER in human induced stem cells can be seen in the Allen Cell Explorer.

3D-view of the ER in U-2 OS cells, visualized by immunofluorescent staining of HSP90B1. Highly expressed single localizing endoplasmic reticulum proteins across different cell lines. Protein disulfide isomerase family A member 3Ĭytochrome P450 family 51 subfamily A member 1 Heat shock protein 90 beta family member 1 Selection of proteins suitable as markers for the endoplasmic reticulum. Highly expressed genes encoding proteins that localize to the ER are listed in Table 2. Proteins that are suitable as markers for the ER can be found in Table 1.
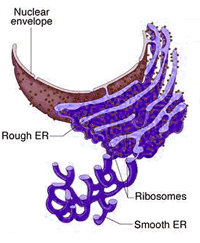
There are also areas that are partially smooth and partially rough, referred to as the transitional ER. In contrast, areas in the tubules are largely devoid of ribosomes, forming the "smooth ER" and is involved in many other functions, depending on cell type ( Friedman JR et al. The sheets with their large surface are studded with ribosomes, forming the "rough ER" and is the primary location for translation. The different membrane-to-lumen ratios in these two domains reflect their different functions. These are often connected by three-way junctions, which result in a polygonal pattern. (2016), made up of flat cisternal, often stacked, sheets and reticulated tubules. The ER is a large dynamic structure ( Schwarz DS et al. Examples of the morphology of the ER in different cell lines, represented by immunofluorescent staining of the protein encoded by LRRC59 in U-2 OS, U-251 MG, and A-431 cells. The structure of the endoplasmic reticulumįigure 3. VAPA may regulate the morphology of the ER by interacting with the cytoskeleton (detected in A-431 cells). STIM1 is a transmembrane protein that is involved in the regulation of calcium ions (detected in A549 cells). ELOVL5 is an ER membrane protein that catalyzes the first and rate limiting reaction in the elongation of long and very long-chain polyunsaturated fatty acids (detected in A-431 cells). Examples of proteins localized to the endoplasmic reticulum. Examples of ER-associated proteins can be found in Figure 1.įigure 1. A Gene Ontology (GO)-based functional enrichment analysis of the ER proteins shows enriched terms for biological processes related to protein synthesis, protein folding, protein modification, mRNA degradation and metabolic processes. Around 54% (n= 282) of the ER proteins localize to other cellular compartments in addition to the ER, the most common ones being the cytosol and vesicles. In the Subcellular Section, 523 genes (3% of all protein-coding human genes) have been shown to encode proteins that localize to the ER (Figure 2). The expanded surface of the ER membrane as well as the distinct composition of the ER lumen provides a platform for various biochemical reactions, but especially for protein biosynthesis and production of lipids. The endoplasmic reticulum (ER) is a delicate membranous network composed of sheets and tubules that spread throughout the cytoplasm and are contiguous with the nuclear membrane.


 0 kommentar(er)
0 kommentar(er)
